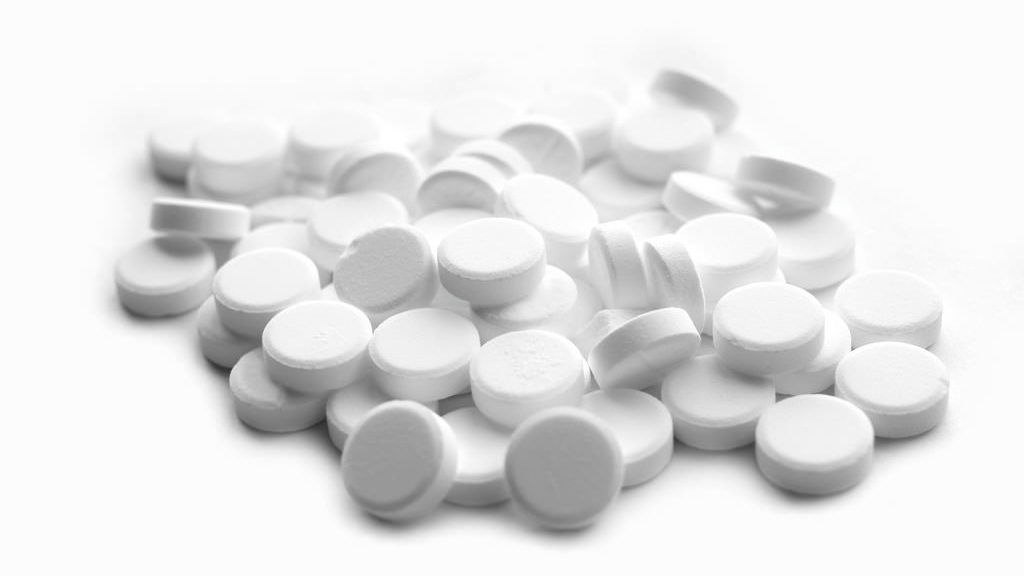
After calls from the Food and Drug Administration to discontinue an opioid product, drugmaker Endo International Plc. has opted to remove its Opana ER opioid painkiller from the market.
As a result of the announcement, shares in the publicly traded firm fell at 3 percent.
Such aftermath wasn't as bad as when the firm's shares fell 12 percent after FDA determined that Opana ER had more risk to overall public health over and perceived benefits. FDA's move, according to Reuters, was also the first time ever request a drug to be removed from the market while citing threats to public health.
"Endo plans to work with FDA to coordinate the orderly removal of Opana ER in a manner that looks to minimize treatment disruption for patients and allows patients sufficient time to seek guidance from their healthcare professionals," an Endo press release on the matter states. "Patients taking Opana ER should discuss treatment options with their prescribing physician at their next visit."
Endo is losing a funding stream that only valued less than 5 percent of the company's overall revenue in 2016. Opana ER raked in $159 million that same year.
The move to remove Opana ER from the market was predated by the call to address the opioid epidemic that has scorched the United States.
Under the new administration, FDA Commissioner Scott Gottlieb placed combating the epidemic as a major priority for the agency. He said that using regulatory strategies to follow through with recommendations and enforcement actions in future situations like this required when certain risks outweigh the benefits of opioid drug use.
The removal of Opana ER also came years after the firm released the drug as an abuse-resistant solution to its original formula, first approved in the early 2000s. The abuse-resistant Opana ER formula was approved in 2012; however, FDA, via the recommendations of a review body, stated that abuse of the drug was still occurring despite prior efforts.
Endo is globally headquartered in Dublin, Ireland with an American corporate office in Malvern, PA.
Should Endo have pulled Opana ER at the request of the FDA? Share your thoughts in the comments.
MORE BUSINESS STORIES ON CAPITALISM.COM:
• Slack Communities for Startup CEOs
• What Houzz Capital Raise, Valuation Could Mean for Home Product Businesses
• New Research Says Sex Appeal Doesn’t Sell As Previously Thought